Debye Specific Heat
By associating a phonon energy
By associating a phonon energy
with the vibrational modes of a solid, where vs is the speed of sound in the solid, Debye approached the subject of the specific heat of solids. Treating them with Einstein-Bose statistics, the total energy in the lattice vibrations is of the form
This can be expressed in terms of the phonon modes by expressing the integral in terms of the mode number n.
Here the factor 3p/2 comes from three considerations. First, there are 3 modes associated with each mode number n: one longitudinal mode and two transverse modes. Then you get a factor of 4p2 from integrating over the angular coordinates, treating the mode number n as the radius vector. Finally you constrain the integral to the quadrant in which all the components of n are positive, giving a factor of 1/8: the product of those is 3p/2.
The usual form of the integral is obtained by making the substitution
and the limit on the integral in terms of x is obtained from
where the constant TD is here introduced. It is called the Debye temperature and is a constant associated with the highest allowed mode of vibration.
When all the constants are put in, the integral takes the form:
The Debye specific heat expression is the derivative of this expression with respect to T. The integral cannot be evaluated in closed form, but numerical evaluation of the integral shows reasonably good agreement with the observed specific heats of solids for the full range of temperatures, approaching the Dulong-Petit Law at high temperatures and the characteristic T3 behavior at very low temperatures.
The specific heat expression which arises from Debye theory can be obtained by taking the derivative of the energy expression above.
This expression may be evaluated numerically for a given temperature by computer routines.
Since the Debye specific heat expression can be evaluated as a function of temperature and gives a theoretical curve which has a specific form as a funtion of T/TD, the specific heats of different substances should overlap if plotted as a function of this ratio. At left below, the specific heats of four substances are plotted as a function of temperature and they look very different. But if they are scaled to T/TD, they look very similar and are very close to the Debye theory.
THEORY
Since a solid can be modelled as a collection of independent oscillators, we can obtain the energy in thermal equilibrium using the Planck distribution function:
where s = 1; 2; 3 are the three polarizations of the phonons and the integral is overthe Brillouin zone.This can be rewritten in terms of the phonon density of states, g(w) as:
The total number of states is given by:
The total number of normal modes is equal to the total number of ion degrees offreedom. For a continuum elastic medium, there are two transverse modes with velocity vtand one longitudinal mode with velocity vl. In the limit that the lattice spacing is very small, a ---> 0, we expect this theory to be valid. In this limit, the Brillouin zoneis all of momentum space, so
In a crystalline solid, this will be a reasonable approximation to g(w) where the only phonons present will be at low energies, far from the Brillouinzone boundary. At high temperatures, there will be thermally excited phonons near the Brillouin zone boundary, where the spectrum is de nitely not linear, so we cannot use the continuum approximation. In particular, this g(w) does not have a nite integral, which violates the condition that the integral should be the total number of degrees of freedom.A simple approximation, due to Debye, is to replace the Brillouin zone by a sphere of radius kD and assume that the spectrum is linear up to kD. In other words, Debyeassumed that:
Here, we have assumed, for simplicity, that vl = vt and we have written D is chosen so that
The T3 contribution to the speci c heat of a solid is often the most important contribution to the measured specific heat.
The high-temperature specific heat is just kB=2 times the number of degrees of free-dom, as in classical statistical mechanics.At high-temperature, we were guaranteed the right result since the density ofstates was normalized to give the correct total number of degrees of freedom. Atlow-temperature, we obtain a qualitatively correct result since the spectrum is linear. To obtain the exact result, we need to allow for longitudinal and transverse velocities which depend on the direction, since rotational invariance is not present.Debye's formula interpolates between these well-understood limits. For lead, which is soft, while fordiamond, which is hard.
ORLANING COLMENARES
ELECTRONICA DEL ESTADO SOLIDO
Visitar mi BLOG:
BIBLIOGRAFIA:
Get news, entertainment and everything you care about at Live.com. Check it out!




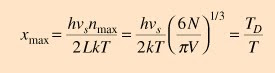











No hay comentarios:
Publicar un comentario