The Bravais lattice are the distinct lattice types which when repeated can fill the whole space. The lattice can therefore be generated by three unit vectors, a1, a2 and a3 and a set of integers k, l and m so that each lattice point, identified by a vector r, can be obtained from:
r = k a1 + l a2 + m a3
In two dimensions there are five distinct Bravais lattices, while in three dimensions there are fourteen. These fourteen lattices are further classified as shown in the table below where a1, a2 and a3 are the magnitudes of the unit vectors and a, b and g are the angles between the unit vectors.
Cubic lattices
Cubic lattices are of interest since a large number of materials have a cubic lattice. There are only three cubic Bravais lattices. All other cubic crystal structures (for instance the diamond lattice) can be formed by adding an appropriate base at each lattice point to one of those three lattices. The three cubic Bravais lattices are the simple cubic lattice, the body centered cubic lattice and the face centered cubic lattice. A summary of some properties of cubic latices is listed in the table below:
Cubic lattices are of interest since a large number of materials have a cubic lattice. There are only three cubic Bravais lattices. All other cubic crystal structures (for instance the diamond lattice) can be formed by adding an appropriate base at each lattice point to one of those three lattices. The three cubic Bravais lattices are the simple cubic lattice, the body centered cubic lattice and the face centered cubic lattice. A summary of some properties of cubic latices is listed in the table below:
Cubic lattices have the highest degree of symmetry of any Bravais lattice. They belong to the (m3m) symmetry group which contains the following symmetry groups and operations:
Note that the (m3m) symmetry group is the highest possible symmetry group associated with a cubic crystal. A limited symmetry of the basis (the arrangment of atoms associated with each lattice point) can yield a lower overall symmetry group of the crystal.
Simple cubic lattice
The simple cubic lattice consists of the lattice points identified by the corners of closely packed cubes.
The simple cubic lattice contains 1 lattice point per unit cell. The unit cell is the cube connecting the individual lattice points. The atoms in the picture are shown as an example and to indicate the location of the lattice points. The maximum packing density occurs when the atoms have a radius which equals half of the side of the unit cell. The corresponding maximum packing density is 52 %.
Body centered cubic lattice
The body centered lattice equals the simple cubic lattice with the addition of a lattice point in the center of each cube.
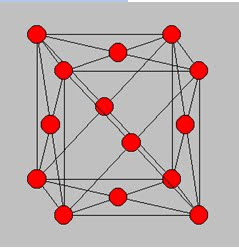
The body centered cubic lattice contains 2 lattice point per unit cell. The maximum packing density occurs when the atoms have a radius which equals one quarter of the body diagonal of the unit cell. The corresponding maximum packing density is 68 %.
Face centered cubic lattice
The face centered lattice equals the simple cubic lattice with the addition of a lattice point in the center of each of the six faces of each cube.
The face centered cubic lattice contains 4 lattice point per unit cell. The maximum packing density occurs when the atoms have a radius which equals one quarter of the diagonal of one face of the unit cell. The corresponding maximum packing density is 74 %. This is the highest possible packing density of any crystal structure as calculated using the assumption that atoms can be treated as rigid spheres.
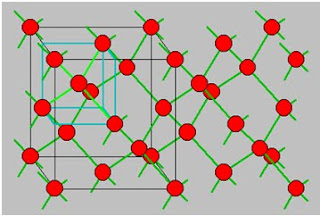
Diamond lattice
The diamond lattice consist of a face centered cubic Bravais point lattice which contains two identical atoms per lattice point. The distance between the two atoms equals one quarter of the body diagonal of the cube. The diamond lattice represents the crystal structure of diamond, germanium and silicon.
The diamond lattice contains also 4 lattice point per unit cell but contains 8 atoms per unit cell. The maximum packing density occurs when the atoms have a radius which equals one eighth of the body diagonal of the unit cell. The corresponding maximum packing density is 34 %.
Zincblende lattice
The zincblende lattice consist of a face centered cubic Bravais point lattice which contains two different atoms per lattice point. The distance between the two atoms equals one quarter of the body diagonal of the cube. The diamond lattice represents the crystal structure of zincblende (ZnS), gallium arsenide, indium phosphide, cubic silicon carbide and cubic gallium nitride.
Orlaning Colmenares
C.I.V.- 18.991.089
Electrónica del Estado Sólido
Referencias Bibliograficas:
Visitar mi BLOG:
Explore the seven wonders of the world Learn more!






No hay comentarios:
Publicar un comentario